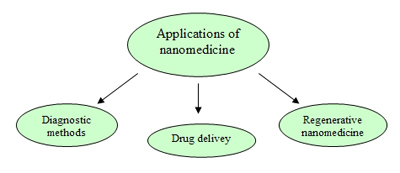
We have already studied in previous entries the long and specialised process that leads to the discovery and selection among thousands of candidates of the lead compound.
Next, I will explain the stage named pre-clinic phase, which is developed between drug discovery and clinical trials. This stage is divided into two parts: one focused on determining the lead compound’s safety and the second based on incorporating the pharmacological complex in a drug delivery system.
There are a wide variety of drug delivery systems, all of them with development, manufacturing, storage and use specific rules. Among the most common and popular ones we find oral medications (pills, capsules, tablets...).
Resuming where we left off in the last entry, we start from an optimised lead compound, which: is capable of binding to one of the therapeutic targets which play a major role in disease progress, has a structure that looks druggable and is apparently non-toxic.
From now on, the pharmaceutical formulator is in charge of defining the best way to administer this compound to its target or ‘action’ site within the organism.
The most effective methodology in support of this process is to keep concentrating on obtaining a broader knowledge of the compound itself, always keeping in mind the type of drug delivery system that will be used. To do this, we must ask a series of questions about its physical and chemical properties: is it soluble in water, in oil or a combination of both?, can it crystallise?, can these crystals reduced in smaller particles?, is it a stable drug from a chemical point of view?, if it is so, how long?, how is it degraded?, does it do in an acidic environment? All these questions must be answered before it is incorporated in an actual dosage form.
From now on, the pharmaceutical formulator is in charge of defining the best way to administer this compound to its target or ‘action’ site within the organism.
The most effective methodology in support of this process is to keep concentrating on obtaining a broader knowledge of the compound itself, always keeping in mind the type of drug delivery system that will be used. To do this, we must ask a series of questions about its physical and chemical properties: is it soluble in water, in oil or a combination of both?, can it crystallise?, can these crystals reduced in smaller particles?, is it a stable drug from a chemical point of view?, if it is so, how long?, how is it degraded?, does it do in an acidic environment? All these questions must be answered before it is incorporated in an actual dosage form.
One of the most basic features that must be known about a drug is its solubility in water. When drugs are given most of them are in contact with some water-based biological fluid since the human body is mostly made up of water.
Solubility is a vital characteristic for the vast majority of drugs, but especially for those that are absorbed in the gastrointestinal (GI) tract. Thus, for example, an aspirin could not be absorbed in the small intestine even if it were split up into smaller fragments if it were not dissolved in the intestinal fluids.
Solubility is a vital characteristic for the vast majority of drugs, but especially for those that are absorbed in the gastrointestinal (GI) tract. Thus, for example, an aspirin could not be absorbed in the small intestine even if it were split up into smaller fragments if it were not dissolved in the intestinal fluids.
In the case that a compound has no, or low solubility in water, the pharmaceutical formulator will try to increase its solubility. As a second alternative, a step back can be taken in this process and the compound can be sent back to a chemical laboratory where a salt form of the drug is synthesised.
The compound solubility not only must be tested in aqueous media, but also in diverse acidic media, due to the fact that gastrointestinal fluids have diverse acidity levels.
In addition to water other non-aqueous solvents used in the final formulation of the drug must be evaluated such as glycerol, ethanol or propylene glycol, as well as solvents used in the manufacturing process like isopropyl alcohol, methanol and acetonitrile.
In addition to water other non-aqueous solvents used in the final formulation of the drug must be evaluated such as glycerol, ethanol or propylene glycol, as well as solvents used in the manufacturing process like isopropyl alcohol, methanol and acetonitrile.
Sources: UTAustinX: UT.4.01x Take Your Medicine - The Impact of Drug Development.
http://indacea.org/desarrollo-de-medicamentos-1/
Valentia Biopharma S.L.
http://www.pharmatechespanol.com.mx/articulo/820.polimeros_avanzados_para_mejora_de_la_solubilidad
http://indacea.org/desarrollo-de-medicamentos-1/
Valentia Biopharma S.L.
http://www.pharmatechespanol.com.mx/articulo/820.polimeros_avanzados_para_mejora_de_la_solubilidad
Read more