First of all, to begin this section I would like to describe a brief overview about the different phases in drug development that we will study in more detail as we move forward.
Drugs are one of the substances that we are more familiar with in our daily life and we consume them more or less often depending on our health condition. However, very few people know their manufacturing process and the huge effort needed so that they can reach the market and our hands.
All around the world there are thousands of research teams analysing diseases and trying to find out their origins and causes. Once causes have been found, biopharmaceutical companies start to investigate how they can act to stop them or even revert their advance. To accomplish this goal tens of thousands of compounds are tested, whose origin can be:
▣ Natural: from substances that already exist in nature like morphin (plant origin), penicillin (microbial origin) or trabectedin (anti-tumor drug of marine origin).
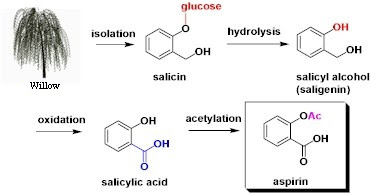
▣ Semisynthetic: compounds obtained in the lab like acetylsalicylic acid, synthesised from willow bark.
▣ Synthetic: compounds like ibuprofen with a complete chemical synthesis.
▣ Biotechnological: these drugs are manufactured with biotechnological techniques where the genetic material of bacteria is modified to produce substances with pharmaceutical interest such as human hormones (insulin) or antibodies.
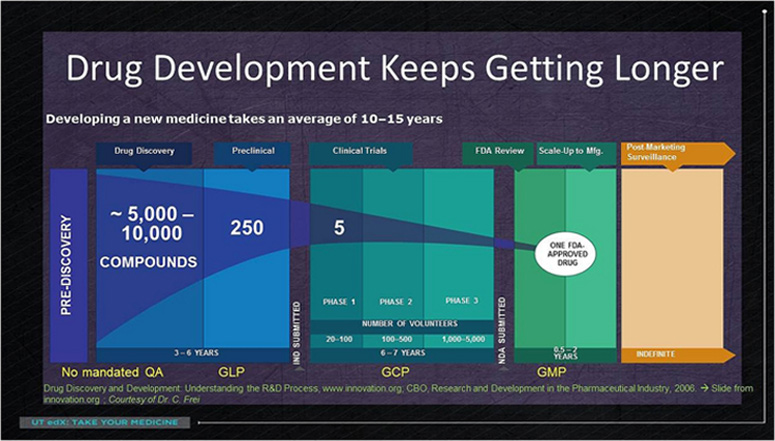
Through a hard and long screening process, that can take over six years, just a few drug candidates are selected (around 10 out of 10,000 initial substances).
All the information about their toxicity and activity in cells and experimental animals is gathered (this phase is called “preclinical phase” where good laboratory practices are followed: GLP). With this information the company requests authorization a drug regulatory agency to initiate the clinical trials. If this authorization is given, the drug candidates are subject to a clinical trial (according to the good clinical practice guidelines: GCP) divided into three stages. Due to the emphasis on safety and effectiveness not all candidates cannot move beyond this long process successfully. These three stages are the following:
All the information about their toxicity and activity in cells and experimental animals is gathered (this phase is called “preclinical phase” where good laboratory practices are followed: GLP). With this information the company requests authorization a drug regulatory agency to initiate the clinical trials. If this authorization is given, the drug candidates are subject to a clinical trial (according to the good clinical practice guidelines: GCP) divided into three stages. Due to the emphasis on safety and effectiveness not all candidates cannot move beyond this long process successfully. These three stages are the following:
▣ In phase 1 the drug is tested on human beings for the first time. Small trials, which are focused on safety and how the drug is distributed in the body, are performed on healthy volunteers.
▣ In phase 2 the trial is conducted at a larger scale on volunteers actually suffering from the disease of interest. In this stage the main concern is drug effectiveness and the right doses.
▣ In phase 3 the trials are carried out on a large number of people in order to enhance the acquired knowledge in the former phase with a larger and broader range of people, involving a lot more money as well.
If the clinical trial succeeds, which takes around 6-7 years, the biopharmaceutical company processes a new application that will be reviewed thoroughly for a long period of time.
Once the evaluation is over, only safe and effective drugs will be authorised for public use, but despite this approval the regulatory agency will keep monitoring the drug in the market.
Not only during the preclinical and clinical stages strict regulations are followed, but also during the manufacturing process. These rules and regulations are known as GMP guidelines (good manufacturing practices).
Although the final result of this process can be as simple as taking a pill with some water twice a day, it requires a total period of time from 12 to 15 years, an investment between $1-$2 billion and thousands of people who are involved to develop a single drug.
Source: UTAustinX: UT.4.01x Take Your Medicine - The Impact of Drug Development.





Your opinion matters