A chemical bond is defined as a force that joins two or more atoms from the same or different element to build more complex structures: molecules. Among the main bonds that form the biological macromolecules, which we will study in-depth later, we can find:
▣ Ionic bonds: between ions with opposite charges. An illustrative example of this kind of bond is represented by sodium chloride molecule (NaCl), commonly known as table salt. This molecule is formed by ionization[1] of sodium atom (Na) and chlorine atom (Cl), and the attraction of resulting ions.
▣ Covalent bonds: the electrons are shared between atoms to form bonds and satisfy the octet rule[2]. Within covalent bonds we can find two subtypes:
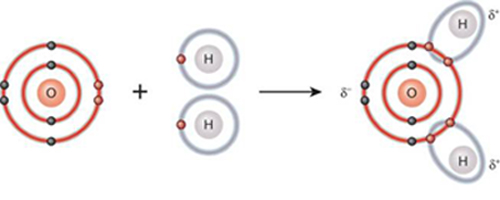
1. Polar covalent bonds: the electrons are unequally shared by the atoms in such a way that they are more attracted by one of the nuclei than by the other (they have different electronegativity). Hence, one side of the molecule is slightly negative (δ-) and the other slightly positive (δ+). An example is water molecule where the oxygen atom is negative charged and the two hydrogen atoms are positive charged.
2. Nonpolar covalent bonds: are formed by two atoms where the electrons are shared equally. Molecular oxygen (O2) is an example where the electrons are distributed evenly.
▣ Hydrogen bonds: weak interactions between hydrogen atoms (slightly positive) and other atoms (usually nitrogen and oxygen) from the same or different molecule. They are in charge of zipping together the two strands of the DNA double helix molecule.
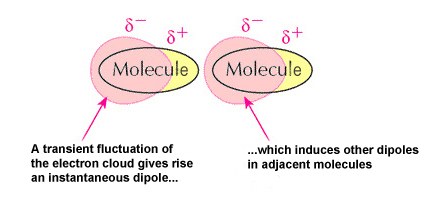
▣ Van der Waals interactions: weak interactions or attractions between molecules due to the fact that two or more of them depend on slight fluctuations of the electronic densities[3], which are not always symmetrical around the atoms. This kind of interaction contributes with ionic, covalent and hydrogen bonds to the three-dimensional structure of proteins.
[1] Process by ions are produced.
[2] It establishes that those atoms with eight electrons in their outermost shell or valence shell are more stable from an energetic point of view.
[3] Probability of finding an electron in a certain region of space.
[2] It establishes that those atoms with eight electrons in their outermost shell or valence shell are more stable from an energetic point of view.
[3] Probability of finding an electron in a certain region of space.
Source: OpenStax College, Biology. OpenStax College. 30 May 2013.






Your opinion matters