Practically all tasks performed by living organisms require energy. Energy is not only needed to perform exercise or demanding tasks, but also when we think, and even when we sleep; its use is, therefore, constant.
Energy is used so that immune system cells can absorb, ingest and break down viruses and pathogenic bacteria. It is also fundamental to eliminate toxins and waste, to transport neurotransmitters and hormones and in the synthesis and chemical breakdown of molecules.
Nutrients and other molecules are imported, metabolised (broken down) and synthesised in other molecules, modified in some cases, transported to other cells and probably distributed throughout the body by using energy.
All cellular processes, such as the building and degradation of complex molecules occur through a series of staged chemical reactions.
These chemical reactions within cells, including those that release and use energy, is called cell metabolism.
These chemical reactions within cells, including those that release and use energy, is called cell metabolism.
Metabolic pathways
A metabolic pathway is a series of interconnected chemical reactions that transform one or several substrate molecules gradually, via a group of metabolic intermediates, into a final product or products.
In the case of sugar metabolism, glucose (a simple sugar) is synthesised from smaller molecules:
While in another different metabolic pathway, glucose is broken down into smaller molecules:
Hence, metabolism consists of building or anabolism (first reaction) and breakdown or catabolism (second reaction).
In the case of sugar metabolism, glucose (a simple sugar) is synthesised from smaller molecules:
6CO2 + 6H2 O + energy → C6 H12 O6 + 6O2
While in another different metabolic pathway, glucose is broken down into smaller molecules:
C6H12O6 + 6O2 → 6CO2 + 6H2 O + energy
Hence, metabolism consists of building or anabolism (first reaction) and breakdown or catabolism (second reaction).
These metabolic pathways do not ocurr spontaneously, but each reaction is enabled or catalysed by specific proteins called enzymes, which catalyse both the reactions that release energy and the reactions that require its use.
Potential, free and activation energy
 When the bonds in molecules are broken down, they release energy. This is known as potential energy. To carry out their function, cells depend on the extraction of that potential energy.
When the bonds in molecules are broken down, they release energy. This is known as potential energy. To carry out their function, cells depend on the extraction of that potential energy.Free energy is a concept that measures the available energy to perform a task. This free energy is modified during chemical reactions (where energy transfers occur) and this change is denominated as Gibbs free energy (ΔG).

If a reaction is spontaneous, this indicates that its products have less energy than its reactives, and vice versa for non-spontaneous reactions.
Regardless of the sort of reaction, all of them however require an initial contribution of energy to reach the transition state[1]. This contribution is known as activation energy.

The value of ΔG can be negative (whereby energy is released). These reactions are called exergonic or spontaneous. On the contrary, if the value of ΔG is positive then the reaction consumes energy. These reactions are called endergonic or non-spontaneous.
If a reaction is spontaneous, this indicates that its products have less energy than its reactives, and vice versa for non-spontaneous reactions.
Regardless of the sort of reaction, all of them however require an initial contribution of energy to reach the transition state[1]. This contribution is known as activation energy.
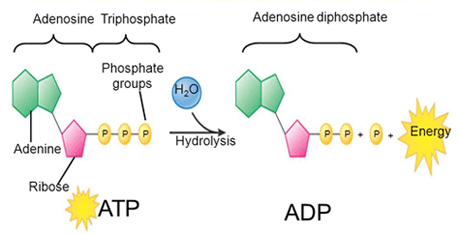
ATP: adenosine triphosphate
ATP is the molecule that provides energy to living cells. It is composed of a nucleotide, a pentose (a five carbon atom monosaccharide) and three phosphate groups. The bonds of these three phosphate groups have a high energy content that when are broken, promote a series of reactions and cell processes.
The energy that is released in the ATP hydrolysis reaction:
The energy that is released in the ATP hydrolysis reaction:
ATP +H2O → ADP + Pi[2] + energía libre (reacción reversible)
Cells use ATP by coupling the exergonic reaction of ATP hydrolysis with endergonic reactions. ATP gives its phosphate group to another molecule during a process known as phosphorylation. This phosphorylated molecule is less stable than its unphosphorylated form, but the added energy after the addition of the phosphate group enables it to perform the necessary endergonic reactions so that the cell can carry out its different functions.
Enzymes
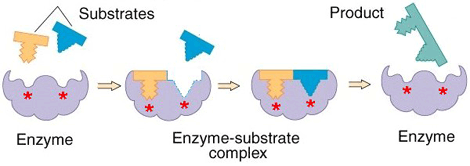
Enzymes (usually one or more polypeptide chain proteins) speed up chemical reactions by diminishing their activation energy requirement.
Enzymes bind to substrates[3] to catalyse reactions in four different ways:
Enzymes bind to substrates[3] to catalyse reactions in four different ways:
▣ Taking part directly in chemical reactions by forming transient covalent bonds with substrates.
▣ Uniting substrates in an optimal orientation.
▣ Providing optimal conditions so that the reaction can take place.
▣ Acting on bond structures so that they can be broken more easily.
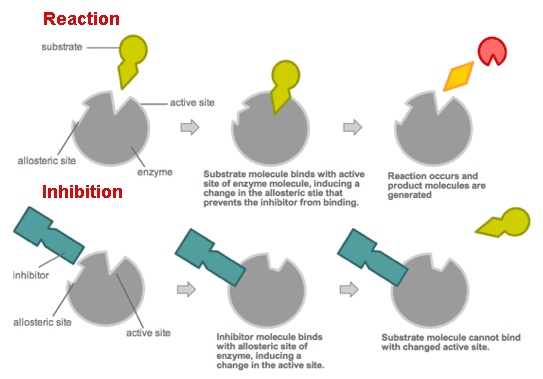
Enzymes are regulated by cell conditions like pH, temperature and their location within the cell (some of them are compartmentalised).
But the most common method by which cells regulate enzymes in metabolic pathways is via feedback inhibition. In this way the products of a metabolic pathway act as inhibitors (normally allosteric[4]) of one or more enzymes involved in the pathway where these products are originated.
But the most common method by which cells regulate enzymes in metabolic pathways is via feedback inhibition. In this way the products of a metabolic pathway act as inhibitors (normally allosteric[4]) of one or more enzymes involved in the pathway where these products are originated.
[1] Very high energy and short duration state that is produced when the reaction reactives approach and experiment a deformation.
[2] Inorganic phosphate group.
[3] Specific chemical reactives on which a specific enzyme performs.
[4] They decrease the enzyme activity by causing structural changes on them, enzyme receptors change and adopt their inactive conformation.
[2] Inorganic phosphate group.
[3] Specific chemical reactives on which a specific enzyme performs.
[4] They decrease the enzyme activity by causing structural changes on them, enzyme receptors change and adopt their inactive conformation.
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://cienciasdejoseleg.blogspot.com.es/2013/03/plicacion-de-las-variables.html
http://teenbiotechchallenge.ucdavis.edu/2010_TBC/Peter%20Wang,%20Clara%20Fannjiang,%20William%20Liu/Chemistry.html
https://mollycools.wordpress.com/2014/03/10/enzymes/
https://commons.wikimedia.org/wiki/File:Allosteric_competitive_inhibition_3.svg
http://cienciasdejoseleg.blogspot.com.es/2013/03/plicacion-de-las-variables.html
http://teenbiotechchallenge.ucdavis.edu/2010_TBC/Peter%20Wang,%20Clara%20Fannjiang,%20William%20Liu/Chemistry.html
https://mollycools.wordpress.com/2014/03/10/enzymes/
https://commons.wikimedia.org/wiki/File:Allosteric_competitive_inhibition_3.svg





Your opinion matters