The energy that enters into our organisms as food is transformed so that cells can use it and perform their functions.
Thus, through a set of metabolic pathways (collectively called cell respiration), the energy of glucose bonds is extracted and turned into an energy form that all living organisms can use.
Thus, through a set of metabolic pathways (collectively called cell respiration), the energy of glucose bonds is extracted and turned into an energy form that all living organisms can use.
Energy in living beings
A living cell does not collect excessive amounts of free energy because it could damage and subsequently destroy it. Hence, cells must store that energy in a safe way and free it only when the need arises, which is achieved by the adenosine triphosphate compound (ATP), which is considered the ‘energy currency’ of cells.
ATP allows the cell to store energy for a short time and to transport it within the cell to facilitate endergonic chemical reactions.
ATP’s structure is made up of an adenosine monophosphate molecule (AMP[1]), which consists of an adenine molecule attached to a ribose molecule and one phosphate group. The addition of a second phosphate group produces adenosine diphosphate (ADP) and the addition of a third phosphate group forms finally adenosine triphosphate (ATP).
ATP’s structure is made up of an adenosine monophosphate molecule (AMP[1]), which consists of an adenine molecule attached to a ribose molecule and one phosphate group. The addition of a second phosphate group produces adenosine diphosphate (ADP) and the addition of a third phosphate group forms finally adenosine triphosphate (ATP).
Life to accomplish its processes and obtain energy, it breaks down ATP into ADP[2] constantly, through the hydrolysis ATP reaction, producing, at the same time, an inorganic phosphate ion:
ATP + H2O ⇔ ADP + inorganic phosphate (Pi)
The water molecule that takes part in this reaction is split into a hydrogen atom and a hydroxyl group. This water is regenerated when a third phosphate is added to the ADP molecule, which reconstitutes, in turn, ATP. This mechanism works, therefore, like a rechargeable battery.
In practically all organisms, the required energy comes from glucose metabolism. This way, ATP is directly connected to the set of exergonic pathways of the catabolism of glucose and the large number of endergonic pathways that supply energy to cells.
The two processes of ATP regeneration used in combination with glucose catabolism are substrate phosphorylation[3] and oxidative phosphorylation.
In the first mechanism, ATP is produced from ADP and a phosphate group from a reactive.
But most of the generated ATP (90%) during the glucose catabolism is derived from a much more complex process, which ocurrs in the mitochondrion: chemiosmosis. During chemiosmosis in which a proton gradient is involved across the mitochondrial membrane.
The production of ATP using this mechanism is called oxidative phosphorylation because of the involvement of oxygen in it.
In the first mechanism, ATP is produced from ADP and a phosphate group from a reactive.
But most of the generated ATP (90%) during the glucose catabolism is derived from a much more complex process, which ocurrs in the mitochondrion: chemiosmosis. During chemiosmosis in which a proton gradient is involved across the mitochondrial membrane.
The production of ATP using this mechanism is called oxidative phosphorylation because of the involvement of oxygen in it.
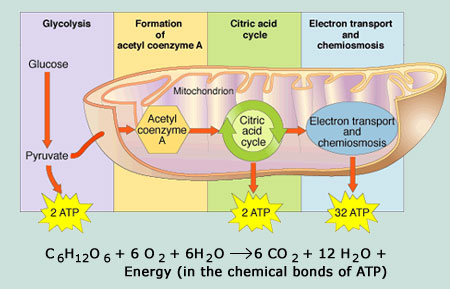
Glycolysis
Glycolysis was probably one of the first metabolic pathways used during the evolution of living beings and it is used by nearly all of them.
Glycolysis is the first point of glucose breakdown for the extraction of energy during the cellular metabolism. It does not require oxygen; it is thus an anaerobic mechanism.
Glycolysis is the first point of glucose breakdown for the extraction of energy during the cellular metabolism. It does not require oxygen; it is thus an anaerobic mechanism.

This process is composed of two steps:
▣ The six carbon atom ring of glucose molecule is divided into two sugar molecules (called pyruvate) with three carbon atoms each one. ATP is needed to cause this separation.
▣ ATP and high energy electrons are extracted from hydrogen atoms, and are attached to NAD+ compounds (oxidised form of the molecule NAD[4]).
The first stage invests two ATP molecules, while the second stage produces four ATP molecules through the substrate phosphorylation mechanism.The final outcome is a net gain of two ATP molecules and two NADH[5] for the cell.
In the event that the cell cannot catalyse the pyruvate molecules, it only gets two ATP molecules from a glucose molecule.
Oxidation of pyruvate and the Citric Acid Cycle (Krebs cycle)
If there is oxygen available, aerobic respiration then takes place following the glycolysis process.
Pyruvate molecules originated at the end of that metabolic pathway are transported into mitochondria where cell respiration takes place. There, pyruvate is transformed into an acetyl group, which is collected and activated by a carrier called coenzyme A (CoA)..
The resultant compound, named acetyl coenzime A, is made up of vitamin B5 (pantothenic acid). This compound is used in a wide range of ways by cells, but its principal function is to distribute the acetyl group that comes from the pyruvate to the following stage in glucose metabolism (the citric acid cycle).
Pyruvate molecules originated at the end of that metabolic pathway are transported into mitochondria where cell respiration takes place. There, pyruvate is transformed into an acetyl group, which is collected and activated by a carrier called coenzyme A (CoA)..
The resultant compound, named acetyl coenzime A, is made up of vitamin B5 (pantothenic acid). This compound is used in a wide range of ways by cells, but its principal function is to distribute the acetyl group that comes from the pyruvate to the following stage in glucose metabolism (the citric acid cycle).

The conversion of pyruvate to an acetyl group removes a CO2 molecule and two high energy electrons. This step occurs twice, so that the CO2 molecule (2CO2) holds two of the six carbon atoms from the initial molecule of glucose. While the NAD+ molecule picks up the electrons forming NADH that conducts those electrons to later pathways in the production of ATP.
At this point, the glucose molecule that entered the cellular respiration mechanism has been completely oxidised and its potential energy has been transferred to electron transporters or has been used to synthesise a few ATP molecules.
Next, the citric acid cycle also begins in the mitochondrial matrix.
Unlike glycolysis, the cytric acid cycle is a closed cycle, in which the last step regenerates the compound used in the first stage.
The cytric acid cycle is an eight step cycle that comprises a series of oxidation-reduction (redox), dehydration, hydration and decarboxylation reactions that produce: two CO2 molecules, one GTP[6]/ATP molecule and the reduced forms of three NADH molecules and one FADH2[7] molecule.
Unlike glycolysis, the cytric acid cycle is a closed cycle, in which the last step regenerates the compound used in the first stage.
The cytric acid cycle is an eight step cycle that comprises a series of oxidation-reduction (redox), dehydration, hydration and decarboxylation reactions that produce: two CO2 molecules, one GTP[6]/ATP molecule and the reduced forms of three NADH molecules and one FADH2[7] molecule.
This cycle is considered an aerobic route because these two last compounds must transfer their electrons to the next pathway of the system, which will use oxygen and will generate ATP.
Some of the intermediate compounds in this cycle can be used to synthesise non-essential amino acids, lipids and sugars that can be energy sources for the metabolic pathways of glucose. Hence, the Krebs cycle is an amphibolic cycle (both anabolic and catabolic).
[1] AMP is one of the nucleotides used in RNA.
[2] Adenosine diphosphate: organic compound made up of adenosine and two phosphate groups.
[3] Addition of a phosphate group to a compound, usually a metabolic intermediate, a protein or ADP.
[4] Nicotinamida adenina dinucleótido: coenzima encontrada en las células vivas, cuya función principal es el intercambio de electrones y protones en las reacciones de producción de energía.
[5] Reduced form of the NAD molecule that therefore, it accepts electrons.
[6] Guanosine triphosphate is another nucleotide required for RNA synthesis and involved in cellular metabolism. Its nitrogenous base is purine guanine.
[7] Reduced form of FAD (flavin adenine dinucleotide: coenzyme that intervenes in metabolic reactions of oxidation-reduction) that accepts two hydrogen atoms.
[2] Adenosine diphosphate: organic compound made up of adenosine and two phosphate groups.
[3] Addition of a phosphate group to a compound, usually a metabolic intermediate, a protein or ADP.
[4] Nicotinamida adenina dinucleótido: coenzima encontrada en las células vivas, cuya función principal es el intercambio de electrones y protones en las reacciones de producción de energía.
[5] Reduced form of the NAD molecule that therefore, it accepts electrons.
[6] Guanosine triphosphate is another nucleotide required for RNA synthesis and involved in cellular metabolism. Its nitrogenous base is purine guanine.
[7] Reduced form of FAD (flavin adenine dinucleotide: coenzyme that intervenes in metabolic reactions of oxidation-reduction) that accepts two hydrogen atoms.
Fuentes: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://table4eversquishycells.pbworks.com/w/page/9947625/How%20Cells%20Get%20Energy
http://bio100.class.uic.edu/lectures/atp_energy.jpg
https://apbionotebook.wordpress.com/chapter-09-cellular-respiration-fermentation/
http://msdoranbiology.weebly.com/notes---cp.html
http://table4eversquishycells.pbworks.com/w/page/9947625/How%20Cells%20Get%20Energy
http://bio100.class.uic.edu/lectures/atp_energy.jpg
https://apbionotebook.wordpress.com/chapter-09-cellular-respiration-fermentation/
http://msdoranbiology.weebly.com/notes---cp.html






Your opinion matters