According to the European regulations, all clinical trials must be performed in accordance with the rules of good clinical practice (GCP) that constitute a guarantee for patient protection and for test results.
That GCP guidelines of the International Conference on Harmonisation (ICH)[1] have been followed since 1996 in Europe; they have also been adopted by the USA and Japan. They are a set of scientific and ethical quality requirements recognised internationally that must be fulfilled by the planning, execution, record and communication of clinical trials where human beings take part.
These rules must guarantee the rights, safety and well-being of trial subjects and the reliability of the results.
These rules must guarantee the rights, safety and well-being of trial subjects and the reliability of the results.
GCP arises from the need to reconciling the subject’s interests (who receives the treatment) and society’s interests in increasing its knowledge about drugs.
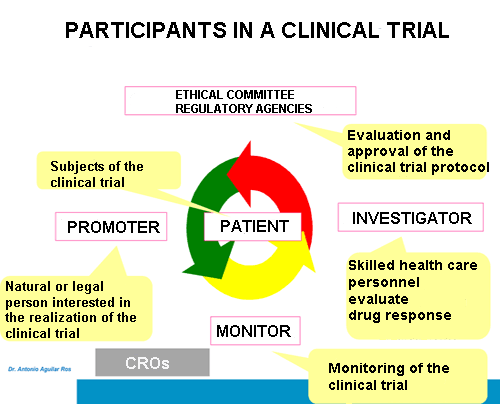
The main actors that participate in a clinical trial are the following:
▣ Promoter: legal entity or person interested in performing a clinical test and responsible for the same. It is usually a pharmaceutical multinational.
▣ Ethical committees: they belong to the centre where the research takes place.
▣ Regulatory agencies: they are usually QUANGOs with government oversight.
These two last participants must approve the protocol and supervise the experimental development. In addition, agencies evaluate the research results to authorise subsequent drug commercialisation.
These two last participants must approve the protocol and supervise the experimental development. In addition, agencies evaluate the research results to authorise subsequent drug commercialisation.
▣ Researchers: carry out or manage the test research. They are normally physicians or health professionals that examine the compound response that is subject of study.
▣ Monitor: responsible for the direct follow-up of the clinical trial execution. Monitor is the intermediary between promoter and researcher and must guarantee the tracking of everything that occurs throughout the experiment.
▣ Patient: subjects who take part in the research and generally suffer from the disease of interest.
On occasion, some companies called CROs (contract research organisations) provide the trial management and monitoring.After the II World War, the Nuremberg Code was promulgated to establish clinical research principles in human beings, which are as follows:
▣ Autonomy: respect the principle for subjects that they are treated as autonomous people to decide freely if they want to take part in the clinical test.
The informed consent[2] signed by the patient is part of the fulfilment of this principle.
The informed consent[2] signed by the patient is part of the fulfilment of this principle.
▣ Well-being: the physician is obligated to do good and to attend to the welfare of the patients, as well as their highest benefit.
▣ Non-malefience: to avoid harmful clinical tests for patients.
▣ Justice: obtained positive results must be shared with the people who suffer from the malady which is the focus of the trial (whether knowledge or new medication). This principle would not be fulfilled, for example, if a country from the third world, where the trial takes place, would not benefit from the treatment because of its high cost.
Phase IV clinical trials
Once a drug has been introduced to the market, studies continue to increase the knowledge about its safety and effectiveness, identify new risks and non-detected adverse response in previous research.
The group of controlled and randomised trials and studies that are performed at this given point is called phase IV of clinical trials.
The group of controlled and randomised trials and studies that are performed at this given point is called phase IV of clinical trials.
The most common epidemiological (observational) studies after the drug authorisation are case-control and cohort studies.
Finally, the Yellow Card Scheme is the system for collecting suspected adverse reactions to drugs. This system is used especially when a medicine has just been marketed and it is mandatory for physicians (and current patients can also fill it out).
These cards consist of several sections among which we find the following:
These cards consist of several sections among which we find the following:
▣ Patient details: first name or initials, age, sex, weight, height…
▣ Data about the suspected medicine causing adverse reactions.
▣ Description of those reactions.
▣ Reporter/clinician details: name, professional address, specialty…
[1] It matches the different regional requirements for the register of pharmaceutical products.
[2] The subject who participates in the clinical trial is informed by this document about the details, advantages and disadvantages of the test with an accessible language, being able to leave it freely at any time.
[2] The subject who participates in the clinical trial is informed by this document about the details, advantages and disadvantages of the test with an accessible language, being able to leave it freely at any time.
Sources: CEU Universidad San Pablo: Farmacología Básica, 2013.
https://www.gov.uk/guidance/the-yellow-card-scheme-guidance-for-healthcare-professionals
https://yellowcard.mhra.gov.uk/the-yellow-card-scheme/
http://www.ich.org/home.htmlhttps://www.gov.uk/guidance/the-yellow-card-scheme-guidance-for-healthcare-professionals
https://yellowcard.mhra.gov.uk/the-yellow-card-scheme/






Your opinion matters