Proteins are the biological macromolecules with the largest variety in structure and functions. We can find thousands of them in a single cell, each performing a unique and specific role. In this way, we find proteins carrying out transport or storage functions, protective, contractile and structural proteins, enzymes or toxins.
All of them have in common that they are made up of structural polymers of amino acids arranged in lineal chains.
All of them have in common that they are made up of structural polymers of amino acids arranged in lineal chains.
Types and functions of proteins
▣ Enzymes: they are known as complex or conjugated proteins, whose essential function is to act as an organic catalyst in biochemical reactions, where they assist to synthesise, reorder or break down different elements on which they perform.
An example are digestive enzymes, like salivary amylase, hydrolyses amylose (starch component).
An example are digestive enzymes, like salivary amylase, hydrolyses amylose (starch component).
▣ Hormones: they control and regulate physiological processes such as metabolism, growth or reproduction. They are chemical-signalling molecules, usually stereoids or small proteins, released by endocrine cells.
Examples include insulin, which is a protein hormone in charge of regulating glucose levels in blood.
Examples include insulin, which is a protein hormone in charge of regulating glucose levels in blood.
Proteins present a wide range of molecular weights and shapes, where shapes are vital to their activities. But these functions can be modified by the exposure to chemicals and changes in pH or temperature, which lead to shape variations, and as a consequence the loss of their functions in a process named denaturation.
Amino acids
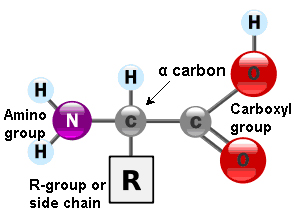
Amino acids are the monomers that constitute proteins. Each one has a structure formed by a central carbon atom, known as α carbon, to which an amino group (NH2), a hydrogen atom and a carboxyl group (COOH) are attached. The name ‘amino acid’ comes from the presence of these two groups in the amino acid basic structure.
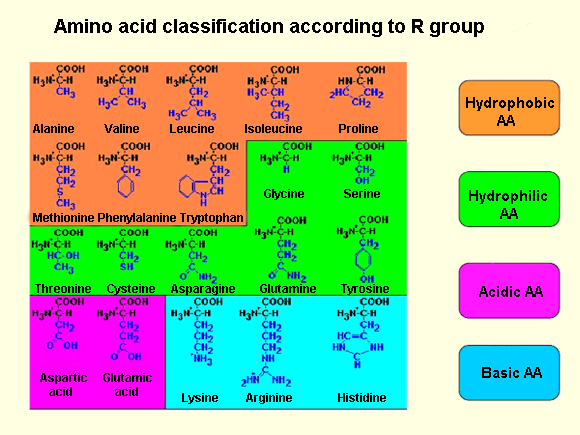
Each amino acid also have another atom or group of atoms joined to the central atom, called R group or side chain which is different for each kind of amino acid. The chemical nature of this chain establishes the type of aminoacid (whether it is polar, nonpolar, basic, acidic…). Thus, we can find basic aminoacids, like arginine and lysine, with a positive charge. Others such as cysteine or serine are polar due to their hydrophilic side chains, or if this chain is hydrophobic we have nonpolar amino acids like alanine or valine.
Out of the twenty different amino acids that form proteins, ten of them are considered essentials, because they are the necessary blocks for the protein construction. However, the human body cannot sinthesise them and must be obtained from the diet.
Amino acids are represented by a single upper case letter or three-letter abbreviation, e.g.: cysteine is symbolised by the letter C or the three letters Cys.
The number and sequence of amino acids ultimately set up the shape, function and size of proteins.
Amino acids are linked by covalent bonds, called a peptide bond, in which the carboxyl group of one amino acid is bonded to the amino group of the adjacent amino acid, releasing a water molecule during the process (dehydration reaction).
These bond products are called peptides and their joining give rise to a polypeptide chain. Each one has a free amino group at the end of the chain, named N terminal or amino terminal, having a C terminal or carboxyl terminal at the other end.
These bond products are called peptides and their joining give rise to a polypeptide chain. Each one has a free amino group at the end of the chain, named N terminal or amino terminal, having a C terminal or carboxyl terminal at the other end.
Often, the terms polypeptide and protein are used interchangeably, although technically a polypeptide is an amino acid polymer, while a protein is a polypeptide or combined polypeptides, each one with distinct shape and unique functions.
Protein structure
As I previously mentioned, protein shape is vital for the role they perform. In order to understand how a protein adopts its shape or final conformation, it is necessary to study the four structural levels in which they are organised: primary, secondary, tertiary and quaternary structures.
Primary structure
The primary structure is defined as the unique sequence of amino acids in a polypeptide chain. For example, the insulin hormone has two polypeptide chains, A and B, formed by unique sequences of 21 and 30 amino acids respectively and joined by two disulfide bonds between the cystein amino acids. There is also a third disulfide bond in the A chain, between two cysteine amino acids, which enables the molecule to fold into the suitable shape.
The unique sequence of every protein is ultimately determined by the genetic coding of that protein. Any alteration in the nucleotide[1] sequence, which constitutes the gene, can produce different amino acids that will be, later, added to the polypeptide chain and consequently affect the activity and structure of the resulting protein.
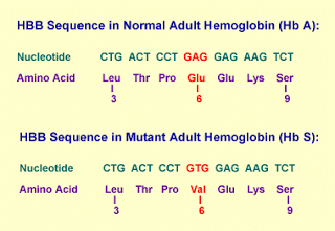
Thus, a single change in one of the 600 amino acids that make up the haemoglobin molecule origins, such a variation in its structure and activity, produces the appareance of sickle cell anaemia[2].Specifically, in the β chain of this protein, glutamic acid is substituted by valine.
More precisely, since every amino acid is made up of three nucleotide bases, those 600 amino acids generate 1,800 bases and sickle haemoglobin, the cause of sickle cell anaemia, arising from a single mutation in those 1,800 bases.
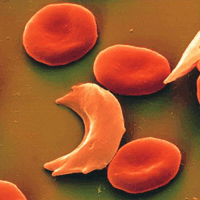
As a result of this, haemoglobin molecules form long fibres that deform the disc or biconcave shape of red blood cells, acquiring a sickle or crescent shape that blocks arteries. This obstruction causes a series of disorders such as headaches, abdominal pain, diziness or breathlessnes so typical of this sickness.
More precisely, since every amino acid is made up of three nucleotide bases, those 600 amino acids generate 1,800 bases and sickle haemoglobin, the cause of sickle cell anaemia, arising from a single mutation in those 1,800 bases.

As a result of this, haemoglobin molecules form long fibres that deform the disc or biconcave shape of red blood cells, acquiring a sickle or crescent shape that blocks arteries. This obstruction causes a series of disorders such as headaches, abdominal pain, diziness or breathlessnes so typical of this sickness.
Secondary structure
Secondary structures are generated from the local folding of polypeptides in certain regions, the most common are the α-helix and β-pleated sheet structures.
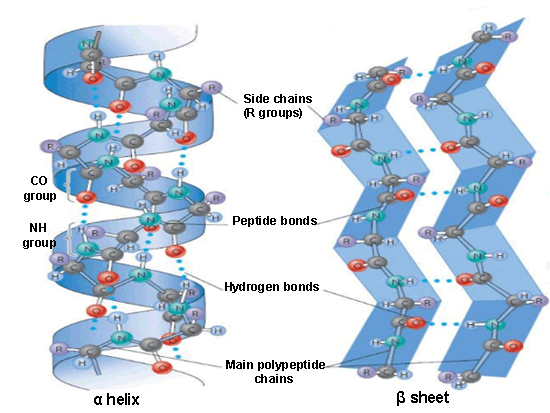 The α-helix is formed from the primary structure by rolling and twisting helically, with 3.6 amino acid residues per turn. They are constituted by hydrogen bonds between the carbonyl group (C=O) of one amino acid and the amino group (N-H) of another amino acid located four positions further in the polypeptide chain.
The α-helix is formed from the primary structure by rolling and twisting helically, with 3.6 amino acid residues per turn. They are constituted by hydrogen bonds between the carbonyl group (C=O) of one amino acid and the amino group (N-H) of another amino acid located four positions further in the polypeptide chain.
 The α-helix is formed from the primary structure by rolling and twisting helically, with 3.6 amino acid residues per turn. They are constituted by hydrogen bonds between the carbonyl group (C=O) of one amino acid and the amino group (N-H) of another amino acid located four positions further in the polypeptide chain.
The α-helix is formed from the primary structure by rolling and twisting helically, with 3.6 amino acid residues per turn. They are constituted by hydrogen bonds between the carbonyl group (C=O) of one amino acid and the amino group (N-H) of another amino acid located four positions further in the polypeptide chain.In the beta formation, amino acids are arranged in a zigzag way, named the ‘pleated sheet’ arrangement. Also it is originated by hydrogen bonds between N-H and C=O groups, but in this case they belong to adjacent chains.
Tertiary structure
The tertiary structure informs how the secondary structure of a polypeptide folds up over itself giving rise to a globular conformation.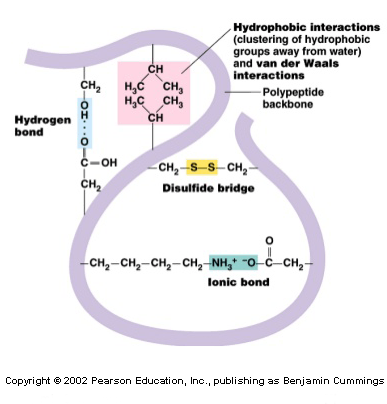
This configuration determines the physicochemical properties of proteins and assists their solubility in water, enabling, this way, it to carry out hormonal enzymatic and transport functions. This structure retains its stability due to the bonds among the R groups of amino acids. These bonds are created from the following types of interactions: disulfide bridges[3], hydrogen bonds, ionic and hydrophobic interactions and Van der Waals forces (to refresh all these concepts, visit the post titled: “Chemical bonds”).
In this arrangement, the nonpolar amino acids are, generally, facing the inside of the protein and the polar amino acids facing the outside, interacting with the surrounding aqueous medium.

This configuration determines the physicochemical properties of proteins and assists their solubility in water, enabling, this way, it to carry out hormonal enzymatic and transport functions. This structure retains its stability due to the bonds among the R groups of amino acids. These bonds are created from the following types of interactions: disulfide bridges[3], hydrogen bonds, ionic and hydrophobic interactions and Van der Waals forces (to refresh all these concepts, visit the post titled: “Chemical bonds”).
In this arrangement, the nonpolar amino acids are, generally, facing the inside of the protein and the polar amino acids facing the outside, interacting with the surrounding aqueous medium.
Quaternary structure
In nature, some proteins are formed from several polypeptides, known as ‘subunits’, and their interaction creates the quaternary structure. These are weak interactions that assist the stabilisation of the overall structure of the protein.
The quaternary structure, usually, gives the protein its function and also creates many crucial biological structures, e.g.: viral capsids[4], microfilaments[5], microtubules[6] and collagen fibres of the connective tissue.
The quaternary structure, usually, gives the protein its function and also creates many crucial biological structures, e.g.: viral capsids[4], microfilaments[5], microtubules[6] and collagen fibres of the connective tissue.
Denaturation and protein folding
Every protein has its own sequence and shape that are maintained through chemical interaction.
If the protein is exposed to chemicals or subjected to variations in pH or temperature, then its shape and structure can be modified without affecting its primary structure. This process is called denaturation, which can be reversible as long as the polypeptide primary structure is invariable during the process, allowing the protein to maintain its activity.
On the other hand, when the process is irreversible, the protein loses its function. This is what albumin (protein of egg white) experiences by being cooked, since it is denatured when it is exposed to high temperatures.
On the other hand, when the process is irreversible, the protein loses its function. This is what albumin (protein of egg white) experiences by being cooked, since it is denatured when it is exposed to high temperatures.
Protein folding plays a vital role for protein functions. This folding is made with the help of a protein family, named chaperones (or chaperonins), present in all cells. They are not part of functional proteins[6], but they bind to them to assist in their folding, their assembly and their cell transport to other parts of the cell where the protein performs its activity.
[1] Monomers of nucleic acids (DNA and RNA).
[2] Crescent or disc-shaped structures.
[3] Strong covalent bond between thiol groups (-SH) in the cysteine amino acid.
[4] Protein coat of virus that protects the viral genome.
[5] Thin fibres of proteins which with microtubules form the cell structure.
[6] Tubular structures of cells. Main component of cytoskeleton in eukaryotic cells.
[7] Those proteins that have a biological activity.
[2] Crescent or disc-shaped structures.
[3] Strong covalent bond between thiol groups (-SH) in the cysteine amino acid.
[4] Protein coat of virus that protects the viral genome.
[5] Thin fibres of proteins which with microtubules form the cell structure.
[6] Tubular structures of cells. Main component of cytoskeleton in eukaryotic cells.
[7] Those proteins that have a biological activity.
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://www.profesorenlinea.cl/Ciencias/ProteinasEstruct.htm
http://study.com/academy/lesson/proteins-iv-higher-order-structure.html
http://quimica.laguia2000.com/conceptos-basicos/cadena-lateral-en-aminoacidos
http://es.slideshare.net/carolinacisdel/biomoleculas-enfermera
http://biologiavfe.blogspot.com.es/2010/04/anemia-falciforme.html
http://sicklecellcurefoundation.org/living-with-sickle-cell-disease/national-sickle-cell-month
http://www.slideshare.net/VijayP7/secondary-structure-prediction-of-proteins
http://www.slideshare.net/thelawofscience/protein-structure-11543259
http://www.mobi4health.ug.edu.pl/wp-content/uploads/2014/10/2ndMSW-Milla-Neffling-Primer.pdf
http://www.profesorenlinea.cl/Ciencias/ProteinasEstruct.htm
http://study.com/academy/lesson/proteins-iv-higher-order-structure.html
http://quimica.laguia2000.com/conceptos-basicos/cadena-lateral-en-aminoacidos
http://es.slideshare.net/carolinacisdel/biomoleculas-enfermera
http://biologiavfe.blogspot.com.es/2010/04/anemia-falciforme.html
http://sicklecellcurefoundation.org/living-with-sickle-cell-disease/national-sickle-cell-month
http://www.slideshare.net/VijayP7/secondary-structure-prediction-of-proteins
http://www.slideshare.net/thelawofscience/protein-structure-11543259
http://www.mobi4health.ug.edu.pl/wp-content/uploads/2014/10/2ndMSW-Milla-Neffling-Primer.pdf






Your opinion matters