In my second to last post (Routes of drug administration), we studied the different drug delivery systems and how one of them is selected to take a drug compound and make it into a drug product.
In the drug development process, researchers often use living organisms to find out if their compounds can go through the cell membranes and carry out their therapeutic function. However, there is a really delicate balance between those therapeutic effects and toxicity in lots of drugs. To avoid this conflict, previously, many studies must have been conducted to select the lead compound with the smallest toxicity and the top therapeutic potency.
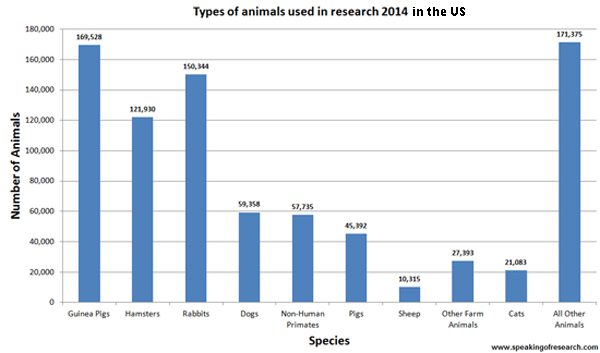
 Many of these evaluations can be assessed on the bench top in cell cultures. However, these experiments are often too simple to predict the compound efficacy in human beings. Hence, more complex living systems, like in rats or mice, are used in order to gain a greater understanding about their efficacy and biological distribution. To do this, researchers have been able to find several ways to model human diseases (e.g. cancer, certain infections and inflammations) in rodents.
Many of these evaluations can be assessed on the bench top in cell cultures. However, these experiments are often too simple to predict the compound efficacy in human beings. Hence, more complex living systems, like in rats or mice, are used in order to gain a greater understanding about their efficacy and biological distribution. To do this, researchers have been able to find several ways to model human diseases (e.g. cancer, certain infections and inflammations) in rodents.
Although these models are not completely reliable indicators of the drug efficacy in humans, they are another tool which helps to choose the lead compound with the greatest chance of success. These models can also be used to determine the optimal dosage of the drug needed to reach its target.
 Many of these evaluations can be assessed on the bench top in cell cultures. However, these experiments are often too simple to predict the compound efficacy in human beings. Hence, more complex living systems, like in rats or mice, are used in order to gain a greater understanding about their efficacy and biological distribution. To do this, researchers have been able to find several ways to model human diseases (e.g. cancer, certain infections and inflammations) in rodents.
Many of these evaluations can be assessed on the bench top in cell cultures. However, these experiments are often too simple to predict the compound efficacy in human beings. Hence, more complex living systems, like in rats or mice, are used in order to gain a greater understanding about their efficacy and biological distribution. To do this, researchers have been able to find several ways to model human diseases (e.g. cancer, certain infections and inflammations) in rodents.Although these models are not completely reliable indicators of the drug efficacy in humans, they are another tool which helps to choose the lead compound with the greatest chance of success. These models can also be used to determine the optimal dosage of the drug needed to reach its target.
Despite the fact that in this phase, the pre-clinical efficacy is not considered as important as the pre-clinical safety, these studies are vital for the pharmaceutical developers to evaluate the likehood of the drug efficacy against a particular human disease. Once the lead compound has been selected and formulated, it is evaluated through animal testing to ensure it is safe for human use.
Although lots of ethical objections have arisen about animal testing, it is the best procedure that scientists and government officials have developed to protect humans against the unanticipated dangers of experimental drugs. In order to ensure the quality and validity of the obtained data in these trials, it has been set a strict group of regulations, called ‘good laboratory practice’ (GLP), which developers have to fulfil. These regulations establish an organisational framework for the new pharmaceutical substances, as well as the evaluation of any toxicity that may happen during examinations.
In addition to an understanding of the potential toxic effects, it is also critical to know how long the drug remains in the bloodstream, how it is metabolised and what tissues it can be collect in.
Next, I will explain how to obtain (on average) the permission of regulatory agencies to subject experimental drugs to clinical studies:
In the US, for instance, if the lead compound overcomes the safety exams, an IND (Investigational New Drug) application is submitted as evidence that the drug is reliable and that it can be put forward for experimentation in humans. Together with this data, the pharmacological product must be manufacturable by a reproducible and controlled method.
In the US, for instance, if the lead compound overcomes the safety exams, an IND (Investigational New Drug) application is submitted as evidence that the drug is reliable and that it can be put forward for experimentation in humans. Together with this data, the pharmacological product must be manufacturable by a reproducible and controlled method.
This stage in the drug development process, which can mean a financial layout of several million dollars for the pharmaceutical developers, can present an insurmountable obstacle for smaller companies and academic researchers.
In principle, the expenditure of such a huge amount of money on a new drug that has not been commercialised yet may appear excessive. Nevertheless, it is absolutely necessary to ensure that the drug will not cause harm to the humans that receive it during the clinical trials.
In principle, the expenditure of such a huge amount of money on a new drug that has not been commercialised yet may appear excessive. Nevertheless, it is absolutely necessary to ensure that the drug will not cause harm to the humans that receive it during the clinical trials.
In the US, as I mentioned earlier, new drug products are given the denomination IND by the regulatory agency FDA (Food and Drug Administration), which does not mean that the new drug has been approved to be marketed, but the drug does then have authorisation to be put under clinical trial.
In order to get an IND, previously an IND application had to be submitted with all the required FDA information guaranteeing that the product is safe and ready to commence clinical trials.
In order to get an IND, previously an IND application had to be submitted with all the required FDA information guaranteeing that the product is safe and ready to commence clinical trials.
During the first stage of clinical trials, the volunteers cannot be exposed, under any condition, to unnecessary health risks. To ensure this, the IND application must contain information about the safety test results of the drug, its manufacturing process and details about the proposed clinical study. As long as the provided information is clear and properly submitted, the FDA opens an IND application. In any case, the FDA could stop the investigation at any point if the study integrity is compromised or the new product turns out to be a health risk.
Sources: UTAustinX: UT.4.01x Take Your Medicine - The Impact of Drug Development.
https://www.msdsalud.es/tu-salud-al-dia/biblioteca-recursos/guias-para-pacientes/proceso-investigacion-desarrollo-aprobacion-
farmaco.html
http://www.thebody.com/content/art16845.html
http://geneticayclonacion.blogspot.com.es/2015_04_01_archive.html
http://speakingofresearch.com/facts/statistics/
http://www.slideshare.net/swati2084/ind-investigational-new-drug-application-and-nda-26468953
https://www.msdsalud.es/tu-salud-al-dia/biblioteca-recursos/guias-para-pacientes/proceso-investigacion-desarrollo-aprobacion-
farmaco.html
http://www.thebody.com/content/art16845.html
http://geneticayclonacion.blogspot.com.es/2015_04_01_archive.html
http://speakingofresearch.com/facts/statistics/
http://www.slideshare.net/swati2084/ind-investigational-new-drug-application-and-nda-26468953







Your opinion matters