In recent years, the definition of a succesful drug has changed. The time when a drug would become a blockbuster is over, since there are no longer illnesses that can be treated with just a drug. At the same time, patients and markets demand more effective medicines.
But this demand turns out to be harder to fulfil because our knowledge about diseases is getting more and more complex. Besides, there are numerous external pressures (from governments, financial institutions, regulatory agencies and even scientists) to achieve the best clinical results.
Then, how is it possible to create new drugs when their development costs increase year after year and it is more difficult to generate them with a clinical benefit for patients?
But this demand turns out to be harder to fulfil because our knowledge about diseases is getting more and more complex. Besides, there are numerous external pressures (from governments, financial institutions, regulatory agencies and even scientists) to achieve the best clinical results.
Then, how is it possible to create new drugs when their development costs increase year after year and it is more difficult to generate them with a clinical benefit for patients?
To overcome this challenge, science has provided us with new tools, such as the ability to map the human genome (at a lower and lower cost), which allows us to understand the expression of different proteins in response to diverse illnesses.
Consequently, personalised medicine, which uses these new kinds of tools, is expected to take the lead in the discovery of new drug development processes and of specific medicines that are more effective and even safer.
Consequently, personalised medicine, which uses these new kinds of tools, is expected to take the lead in the discovery of new drug development processes and of specific medicines that are more effective and even safer.
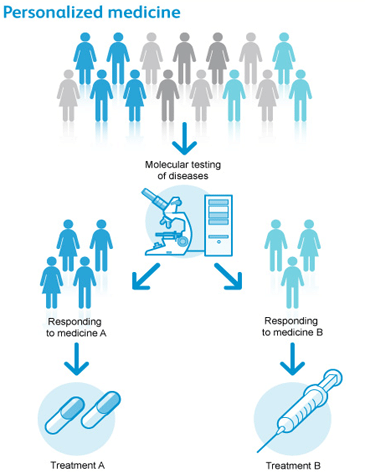
But, how is a personalised drug defined? Personalised medicine is that medical treatment tailored to the features of each and every patient.
It is expected that personalised drugs will be prescribed only to those for whom there will be a known and personal benefit.
In this way, not only the capacity to treat diseases improves, but also, the associated savings in prescribing in a targeted way.
It is expected that personalised drugs will be prescribed only to those for whom there will be a known and personal benefit.
In this way, not only the capacity to treat diseases improves, but also, the associated savings in prescribing in a targeted way.
Within personalised medicine, we find three categories:
▣ Diagnostics: these products can diagnose the risk of suffering from a specific disease, the real existence of a disease in an individual, or even guide the treatment for those people who have already been diagnosed.
▣ Companion diagnostics: : these products work in tandem with pharmaceutical treatments, indicating to the physician the type of drugs that a particular patient should receive.
▣ Screening tools: they are able to detect diseases in their early stages, which allows quick interventions with the hope of diminishing the effects caused by illnesses.
Ideally, it would be interesting to find a way of evaluating a person’s vulnerability to a disease or their response to a certain treatment even before administering it.
With the help of personalised medicine, we could know beforehand which people would respond positively to a specific drug and those that would not; which people would suffer grave side effects and those that would not; which people would need that medicine at an early stage and those who would need it at a later stage. All with the goal of accomplishing the best possible results in each patient.
With the help of personalised medicine, we could know beforehand which people would respond positively to a specific drug and those that would not; which people would suffer grave side effects and those that would not; which people would need that medicine at an early stage and those who would need it at a later stage. All with the goal of accomplishing the best possible results in each patient.
Personalised medicine has experienced spectacular growth in the last few years, from 13 main products in the market in 2006 to 72 products in the year 2011 and sales of 30 billion dollars in the USA.
Presently, 94% of biopharmaceutical companies are researching personalised medicine. They are aware that it can be used both as a research tool as well as to improve a drug’s potential.
Presently, 94% of biopharmaceutical companies are researching personalised medicine. They are aware that it can be used both as a research tool as well as to improve a drug’s potential.
Among the personalised medicine products that physicians use currently to treat their patients are the following:
▣ BRACAnalysis tests that identify potential mutations in the genes BRCA1 and BRCA2 responsible for most ovary and breast inherited cancers. Depending upon the presence or not of these mutations, the patient’s likelihood of developing these types of cancer in the future is determined. According to that, other relatives are guided and advised about what steps and decisions to make.
▣ Oncotype DX is a 21gene test that predicts a patient’s likelihood of benefiting from chemotherapy and the risk of a tumour recurrence in the following ten years.
▣ PreDX is a blood test that examines the levels of seven protein markers associated with diabetes. These markers are related to fat tissue quality, inflammatory conditions of the pancreas and glucose metabolism.
Some of the current benefits from personalised medicine include:
▣ A 34% reduction of chemotherapy in those women who suffer from breast cancer and prior to these treatments they have been undergone genetic testing.
▣ Up to 17,000 strokes can be prevented every year in those patients who are prone to cerebrovascular disorder by giving the proper dose of warfarin[1] provided that a genetic test is carried out.
▣ Over 600 million dollar savings for the health care system in the USA if patients with metastatic colorectal cancer undergo the KRAS gene[2] test before receiving their treatments.
▣ Development of new diagnostic tools that can help in the drug development process. Thus, if pharmaceutical companies know in advance what patients will respond better to a clinical trial, better results will be obtained in that study.
We are still in the early stages of personalised medicine, which will provide us with a better understanding about disease conditions, minimise side effects, and identify new drug targets.
By incorporating it into our experiments and research methods, we will also identify new innovations related to the drug discovery process.
By incorporating it into our experiments and research methods, we will also identify new innovations related to the drug discovery process.
[1] An anticoagulant (blood thinner) used in the prevention of thrombosis and thromboembolism.
[2] Gene that produces KRAS protein, which takes part in many cell signalling patways, cell growth and apoptosis.
[2] Gene that produces KRAS protein, which takes part in many cell signalling patways, cell growth and apoptosis.
Sources: UTAustinX: UT.4.01x Take Your Medicine - The Impact of Drug Development.
http://www.patia.com.mx/braca.html
https://breast-cancer.oncotypedx.com/es-MX/Professional-Invasive/WhatIsTheOncotypeDXBreastCancerTest.aspx
http://www.tuorigen.cl/index.php/preguntas-frecuentes
http://www.cancer.gov/espanol/publicaciones/diccionario?cdrid=652256
http://www.lampadia.com/analisis/tecnologia/medicina-del-futuro-despliegue-de-un-nuevo-paradigma/
http://www.patia.com.mx/braca.html
https://breast-cancer.oncotypedx.com/es-MX/Professional-Invasive/WhatIsTheOncotypeDXBreastCancerTest.aspx
http://www.tuorigen.cl/index.php/preguntas-frecuentes
http://www.cancer.gov/espanol/publicaciones/diccionario?cdrid=652256
http://www.lampadia.com/analisis/tecnologia/medicina-del-futuro-despliegue-de-un-nuevo-paradigma/






Your opinion matters