Among the different functions of the plasma or cell membrane, the most basic is to establish the cell’s limits and keep it functional.
The membrane is selectively permeable. Consequently some elements can enter and exit the cell freely, whereas others need a specialised structure or even energy to accomplish this.
The membrane is selectively permeable. Consequently some elements can enter and exit the cell freely, whereas others need a specialised structure or even energy to accomplish this.
The most significant transport routes that the cell uses through its plasma membrane are:
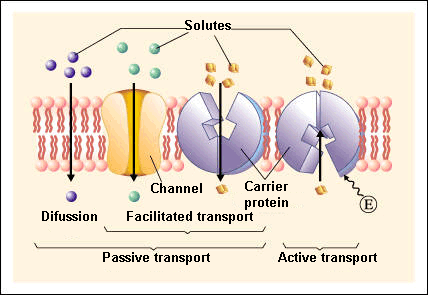
Passive transport
Passive transport is a natural phenomenon where the cell does not need to use any sort of energy to move substances across the plasma membrane.
In passive transport, the substances pass from a high concentration region to a low concentration region.The various mechanisms of passive transport are:
▣ Diffusion: a single substance moves from a high concentration area to a lower one until the concentration is equalised on both sides.
A variation of diffusion is the filtration process where materials move through the membrane according to their concentration gradient[1]. At times, the diffusion rate is increased by pressure causing materials to be filtered much more quickly. This is the process that takes place in kidneys where the blood pressure forces big amounts of water and substances dissolved in it (solutes) from the bloodstream to the renal tubules.
A variation of diffusion is the filtration process where materials move through the membrane according to their concentration gradient[1]. At times, the diffusion rate is increased by pressure causing materials to be filtered much more quickly. This is the process that takes place in kidneys where the blood pressure forces big amounts of water and substances dissolved in it (solutes) from the bloodstream to the renal tubules.
▣ Facilitated transport: diffusion is carried out by the membrane with the help of its embedded proteins, which act like shields for these substances (polar molecules, ions) against the membrane’s repellent forces, facilitating their diffusion inside the cell.
▣ Osmosis: is a special case of diffusion in which water, not material, is carried across the membrane. Therefore, the membrane limits the diffusion of solutes in water. This process is mediated by a set of specific proteins called aquaporins.
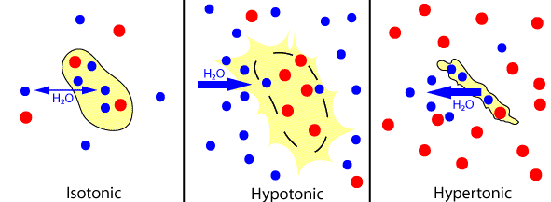
Depending on the relationship between the cell osmolarity[2] and the osmolarity of the surrounding extracellular fluid, we can identify three solution states:
▣ Hypotonic solutions: the osmolarity of the extracellular fluid is lower than the osmolarity of the fluid inside the cell, therefore water penetrates the cell.
▣ Hypertonic solutions: the osmolarity of the extracellular fluid is higher than the cytoplasm osmolarity, hence water leaves the cell.
▣ Isotonic solutions: both the cell and the extracellular fluid have the same osmolarity. As a consequence, there is a constant exchange of water between both regions.
Active transport
Active transport, also known as pumps, requires the use of energy in the form of adenosine triphosphate (ATP). This energetic requirement is necessary because the substance is moving against a concentration gradient (i.e., the substance concentration in the cell is higher than in the extracellular fluid or vice versa).
By this mechanism a wide range of different sized molecules can be carried.
By this mechanism a wide range of different sized molecules can be carried.
In living organisms, there are not only simple concentration gradients but also electrical gradients that indicate a charge difference across the cell membrane.
The cell interior is negative electrically in comparison to the extracellular fluid, which has a higher concentration of K+ (potassium ions) and a lower concentration of Na+ (sodium ions) than the extracellular fluid.
Consequently, the electrical and concentration gradients of Na+ tend to lead these ions into the cell.
The K+ ion situation is more complex; while its electrical gradient also tends to drive these ions towards the interior of the cell, its concentration gradient leads out of the cell.
Consequently, the electrical and concentration gradients of Na+ tend to lead these ions into the cell.
The K+ ion situation is more complex; while its electrical gradient also tends to drive these ions towards the interior of the cell, its concentration gradient leads out of the cell.
These combined gradients of electrical charge and concentration that affect an ion are named electrochemical gradient.
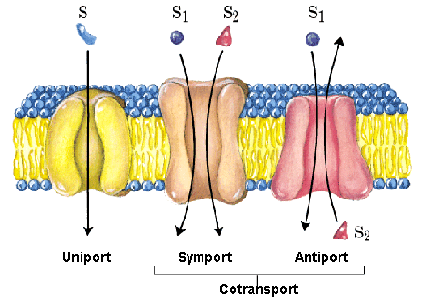
A notable adaptation of the cell membrane for active transport is the presence of specific transport proteins (pumps) that enable the movement of materials on both sides of the membrane. These proteins or transporters are classified into three types:
- Uniporters: they carry a specific molecule or ion in just one direction.
- Symporters: they transport two different ions or molecules in the same direction.
- Antiporters: they also transport two different molecules or ions but in opposite directions.
Primary active transport
This type of transport works with the active transport of K and Na and enables secondary active transport to occur at later stage.
One of the most important mechanisms of active transport in animal cells is the Na+ -K+ pump that maintains the electrochemical gradient and the appropiate concentrations of Na+ and K+ in cells.
During this process for every three Na+ ions that are expelled from the cell, two K+ ions penetrate into the cell, therefore the interior of the cell is slightly more negative than its exterior. This charge difference is vital to create the necessary conditions for the second process.
During this process for every three Na+ ions that are expelled from the cell, two K+ ions penetrate into the cell, therefore the interior of the cell is slightly more negative than its exterior. This charge difference is vital to create the necessary conditions for the second process.
The Na-K pump belongs to the electrogenic pump category, which is a kind of ionic pump that generates a potential electrical charge difference on both sides of the cell membrane.
Secondary active transport (co-transport)
Via this process Na+ ions and other compounds penetrate into the cell taking advantage of the electrochemical gradient created in the previous step.
In this class of active transport a solute that moves against the concentration gradient is co-transported with other solutes that move in favour of its concentration gradient.
Thanks to this method, many diverse molecules, like amino acids or glucose molecules, can enter the cell.
This process is also used to store hydrogen ions of high energy in mitochondria to produce ATP.
In this class of active transport a solute that moves against the concentration gradient is co-transported with other solutes that move in favour of its concentration gradient.
Thanks to this method, many diverse molecules, like amino acids or glucose molecules, can enter the cell.
This process is also used to store hydrogen ions of high energy in mitochondria to produce ATP.
Bulk transport
In addition to small ions and molecules, cells must also assimilate and eliminate larger size molecules and particles and in order to achieve it they need a supply of energy. However, these molecules are so large that not even with the supply of energy can they pass through the plasma membrane.Hence, the cell uses the following mechanisms:
Endocytosis
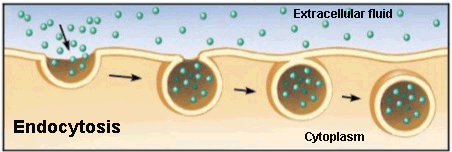
Endocytosis is a type of active transport where particles, such as large molecules, parts of cells and even entire cells are enclosed by invagination of the membrane forming a vesicle whose content is carried from the outside to the inside of the cytoplasm.
Listed are the various endocytosis:
▣ Phagocytosis: is the process in which cells, or large parts are ‘consumed’ by a cell. This is the method used by a particular type of white cells called neutrophils. These immune system cells surround, cover and destroy the microrganisms that invade our body.
▣ Pinocytosis: the cell captures the molecules that are needed from the extracellular fluid. The cell membrane invaginates that unwelcome cell, creating a vesicle around the liquid of the external fluid, which is ‘consumed’ and released in the cytoplasm.
For example, the maturation egg process in the uterus is carried out by pynocytosis when the ovule takes in the nutrients that its supply cells release.
For example, the maturation egg process in the uterus is carried out by pynocytosis when the ovule takes in the nutrients that its supply cells release.
▣ Receptor-mediated endocytosis: uses receptor proteins from the cell membrane that have a specific affinity to binding to particular substances.
Some human diseases are caused by the malfunction of this mechanism. It is the case of familial hypercholesterolemia, in which the receptors of bad cholesterol are faulty or completely absent.
Exocytosis
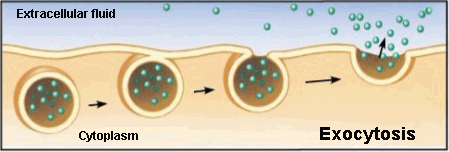
Exocytosis is the opposite to endocytosis in which the cell expels material from its interior to outside.
This waste material is covered with a membranous sac that fuses to the plasma membrane and then opens to release its content into the extracellular space.
The neurotransmitter secretion by vesicles in the synaptic gap of neurons follows this process.
[1] Different concentration of molecules between two regions.
[2] It describes the overall concentration of solute in a solution.
[2] It describes the overall concentration of solute in a solution.
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://www.educ.ar/sitios/educar/recursos/ver?id=14378
http://sevendaysperweek.blogspot.com.es/2015/09/spm-biology-3-movement-of-substances_20.html
http://cbc.arizona.edu/classes/bioc462/462a/NOTES/LIPIDS/transport.html
Nelson & Cox, Lehninger Principles of Biochemistry, 3rd ed., 2000
http://es.slideshare.net/exitoinevitable/clulas-31270712
https://micro.magnet.fsu.edu/cells/endosomes/endosomes.html
http://www.educ.ar/sitios/educar/recursos/ver?id=14378
http://sevendaysperweek.blogspot.com.es/2015/09/spm-biology-3-movement-of-substances_20.html
http://cbc.arizona.edu/classes/bioc462/462a/NOTES/LIPIDS/transport.html
Nelson & Cox, Lehninger Principles of Biochemistry, 3rd ed., 2000
http://es.slideshare.net/exitoinevitable/clulas-31270712
https://micro.magnet.fsu.edu/cells/endosomes/endosomes.html






Your opinion matters