Those compounds that have passed the preclinical phase are tested in human beings (clinical research).
This kind of research has significant associated issues of a scientific, ethical, social and economic nature.
This kind of research has significant associated issues of a scientific, ethical, social and economic nature.
In all developed societies it is mandatory to prove the efficacy and safety of drugs before commercialising them, a task that is fulfilled by clinical trials.
Clinical trials are defined as research studies in human beings to determine or confirm clinical, pharmacological, pharmacodynamic effects and/or detect adverse reactions and/or study the absorption, distribution, metabolism and excretion of a drug in order to establish its safety and efficacy.
Clinical tests are based on documentation, both national and international regulation and a series of ethical codes, declarations and international conventions.
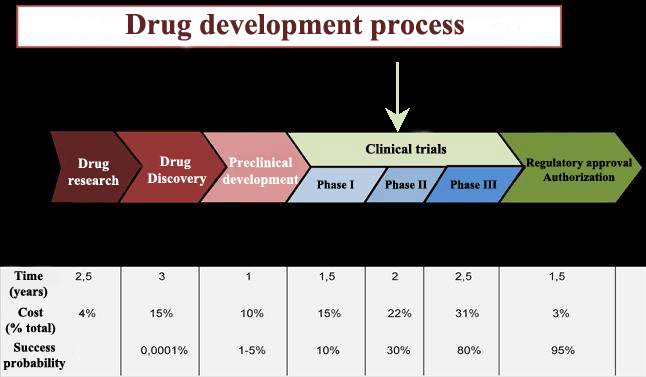
Over the last fifty years hundreds of thousands of compounds have been produced, but only 10% of them have passed clinical evaluation, and even within this group some of them were withdrawn after being put on the market due to safety and efficacy reevaluations.
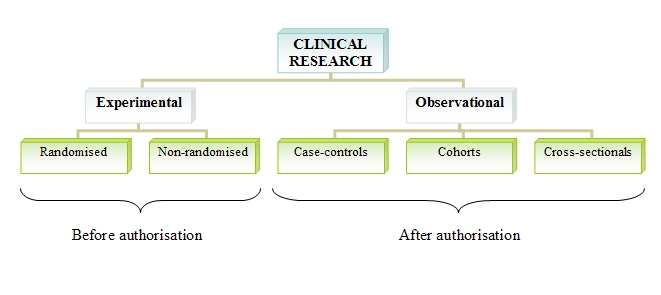
The methodology of clinical research can be carried out via one of the following procedures:
▣ Experimental: In experimental tests, researchers design the study, select the patients, establish the treatments and variables to study.
▣ Observational: Researchers are mere observers, without altering or intervening the habitual clinical practice.
Within the experimental tests, we find:
▣ Randomised trials. In these trials, patients are allocated to one treatment or another (often a placebo) in a random way. This is the procedure demanded by regulatory agencies to avoid bias in data interpretation before giving the authorisation to market the drug.
▣ Non-randomised trials. Here, patients are not allocated randomly.
Among the observational studies to develop the complete efficacy and safety profile of a drug after its commercialisation, we have:
▣ Case-control studies. Participants are chosen according to they have or not a certain disease and it is investigated if they were exposed or not to a factor of interest. The exposed group and the control group are compared.
▣ Cohort studies. The frequency of a sickness is compared between two populations, one exposed to a risk factor and the other population are not.
▣ Cross-sectional studies. They examine the relationship between an illness and a set of variables in a certain population at a given point in time.
Clinical trials chronologically go through three phases until achieving marketing authorisation for the drug. Once the drug is commercialised, it begins its phase IV, which lasts the whole lifespan of the drug. In Phase IV the drug can be removed from the market if undesired effects, not detected in the three previous stages, are identified.
These new side effects can be discovered when the drug is distributed among thousands of patients.
These new side effects can be discovered when the drug is distributed among thousands of patients.
The measured efficacy in these real conditions is called effectiveness to distinguish it from the efficacy measured prior to drug distribution on the market.
Sources: CEU Universidad San Pablo: Farmacología Básica, 2013.
http://cmidd.northwestern.edu/about/drug-discovery-and-development-process/
http://indacea.org/desarrollo-de-medicamentos-1/
http://cmidd.northwestern.edu/about/drug-discovery-and-development-process/
http://indacea.org/desarrollo-de-medicamentos-1/






Your opinion matters