 Over the last few years, imaging diagnostics has significantly increased its relevance such that they have become an indispensable tool to diagnose innumerable diseases such as neurological syndromes, cardiovascular disorders or cancer.
Over the last few years, imaging diagnostics has significantly increased its relevance such that they have become an indispensable tool to diagnose innumerable diseases such as neurological syndromes, cardiovascular disorders or cancer.The convergence between imaging diagnostics and nanotechnology enables the creation of a new series of tracers and contrast agents with a higher resolution and sensitivity, which allows illness detection at earlier stages at cellular or even molecular level. This advance makes the quick application of treatment possible, thus increasing the chances of treatment and survival.
Imaging diagnostic nanosystems are mainly based on three types of nanoparticles: semiconductor, metal and magnetic.
Semiconductor nanoparticles
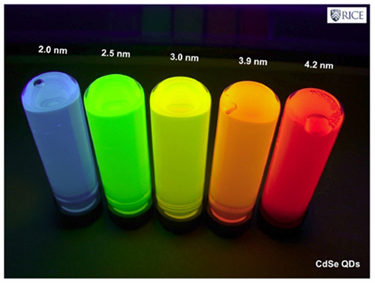
This first sort of nanoparticles, also called quantum dots, consist of semiconductors[1], whose size has been reduced to a few nanometres (1-10 nm). This produces changes in their electronic arrangement, which means they lose their distinctive band structure.
Because of this new structure, their optical response (specifically their fluorescence) is going to be closely related to the size variation of these nanoparticles. Consequently, these quantum dots emit light in a broad range of wavelengths (colours), depending on their size and not the material they are made of, which turns them into excellent biological markers.
Because of this new structure, their optical response (specifically their fluorescence) is going to be closely related to the size variation of these nanoparticles. Consequently, these quantum dots emit light in a broad range of wavelengths (colours), depending on their size and not the material they are made of, which turns them into excellent biological markers.
While a wide variety of semiconductor materials have been examined, the most commonly used are cadmium selenide (CdSe) and cadmium telluride (CdTe), because in addition to be manufactured on a large scale, their sizes can be controlled perfectly. This enables us to see emission bandwiths with a full spectrum of different colours over a long lifetime.
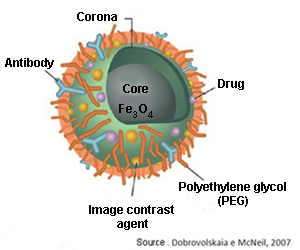
But the semiconductor nanoparticles must also get to their defined targets. To achieve that, the quantum dots must be coated with polymers, like polyethylene glycol (PEG), which makes them invisible to macrophages and prevents immune system cells degrading and digesting them before reaching their target.
The next step is to make these nanoparticles able to identify their targets through bioreceptors (antibodies) that are bound to their surface, and these in turn will be bound to the specific antigens[2] of the target cells (e.g.: cancer cells). The bioreceptors of quantum dots produce a biomolecular recognition reaction when they meet these antigens, being accumulated in that area, and they are observed by using ultraviolet light due to the peculiar fluorescence that they emit.
Lastly and once they have fulfilled their role, the quantum dots must be purged from the organism to avoid unwanted side effects. It seems that the quantum dots are excreted through the kidneys and the liver without difficulties in animal testing, but to be tested in humans some problems related to the aggregation process must be resolved before receiving authorisation from the health agencies for commercialisation.
Metal nanoparticles
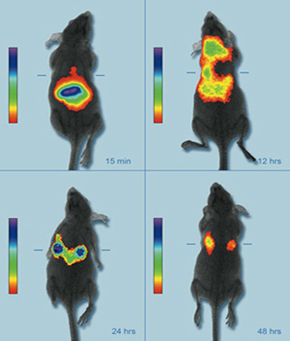 The fact that metal nanoparticles have a resonance frequency (their colour) dependent on their size and shape makes them a second option for contrast agents.
The fact that metal nanoparticles have a resonance frequency (their colour) dependent on their size and shape makes them a second option for contrast agents.This property provides enables them to be manufactured to absorb or reflect light in the spectrum of interest. Thus, gold nanoparticles can be designed to absorb or reflect light in the near-infrared band (700-900 nm) as biological tissues are more transparent in that electromagnetic spectrum band. Using techniques like optical coherence tomography (OCT)[3], 3D maps are obtained of the areas where the nanoparticles are gathered.
Magnetic nanoparticles
A third alternative is magnetic nanoparticles (iron oxides like magnetite: Fe3O4), with the ability to increase the contrast in magnetic resonance imaging (MRI) tests.
 Their transport through the organism can be carried out with the use of an external magnetic field (like an electromagnet) taking advantage of their magnetic properties. They may replace the old fashioned markers made of heavy metals in the near future, because they have lower toxicity.
Their transport through the organism can be carried out with the use of an external magnetic field (like an electromagnet) taking advantage of their magnetic properties. They may replace the old fashioned markers made of heavy metals in the near future, because they have lower toxicity.
 Their transport through the organism can be carried out with the use of an external magnetic field (like an electromagnet) taking advantage of their magnetic properties. They may replace the old fashioned markers made of heavy metals in the near future, because they have lower toxicity.
Their transport through the organism can be carried out with the use of an external magnetic field (like an electromagnet) taking advantage of their magnetic properties. They may replace the old fashioned markers made of heavy metals in the near future, because they have lower toxicity.Both magnetic and metal nanoparticles use identical or very similar methods to the ones described for the quantum dots in order to avoid degradation by the immune system, thus enabling them to locate and bind to the target tissues and cells.
Imaging nanosystems belong to the in vivo diagnostic category, therefore they must be injected into the human body. This implies, as we have seen, potential drawbacks (biocompatibility, sophisticated design) that have to be resolved to ensure an effective and safe use in the organism, minimising undesirable side effects.
[1] Material that behaves as an insulator or a conductive material depending on several factors such as environment temperature, pressure, surrounding electrical and magnetic fields or the incident radiation.
[2] External or toxic substances for the body (generally proteins) that lead to antibody production and causing an immune response.
[3] Non-invasive imaging test that uses light waves to take cross section images.
[2] External or toxic substances for the body (generally proteins) that lead to antibody production and causing an immune response.
[3] Non-invasive imaging test that uses light waves to take cross section images.
Sources: Informe de vigilancia tecnológica: nanomedicina. Fundación para el conocimiento madri+d. CEIM. José Manuel González, Marta López,
Gema Ruiz.
Nanomedicina: aplicación de la nanotecnología en la salud. Laura M. Lechuga. Grupo de Nanobiosensores y Aplicaciones Bioanalíticas
Centro de Investigación en Nanociencia y Nanotecnología (CIN2). CSIC
http://www1.radiology.ucsf.edu/research/labs/hyperpolarized-mri-tech-2/facilities_equipment
http://www.osiconference.org/osi2015/presentations/Tu2.3%20Zahn.pdf
http://www.nanowerk.com/news/newsid=7441.php (Image: Penn State)
http://slideplayer.com.br/slide/74350/
Gema Ruiz.
Nanomedicina: aplicación de la nanotecnología en la salud. Laura M. Lechuga. Grupo de Nanobiosensores y Aplicaciones Bioanalíticas
Centro de Investigación en Nanociencia y Nanotecnología (CIN2). CSIC
http://www1.radiology.ucsf.edu/research/labs/hyperpolarized-mri-tech-2/facilities_equipment
http://www.osiconference.org/osi2015/presentations/Tu2.3%20Zahn.pdf
http://www.nanowerk.com/news/newsid=7441.php (Image: Penn State)
http://slideplayer.com.br/slide/74350/





Your opinion matters