The cell cycle is a set of ordered events that involve the cell growth and division to produce two new daughters cells.
Prior to cell division, cells go through a series of stages of growth, DNA replication and division, carefully regulated, with the objective of creating two identical cells.
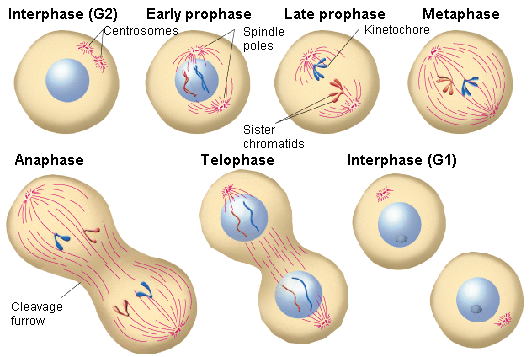
The two main phases of the cell cycle are the interphase and the mitotic phase. During the first stage, the cell grows and replicates its DNA. While, in the mitotic phase, the replicated DNA and the cytoplasmatic content come apart and the cell divides.
Interphase
The interphase is the longest period of the cell cycle, it occurs between two mitosis and we can distinguish the following phases within it:
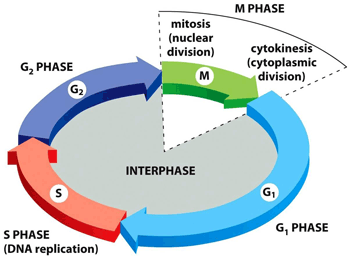
▣ G1: the cell grows, reaching its critical size. RNA and proteins are synthesised and the centrosome is duplicated. If conditions are appropiate, the cell passes the restriction point (a G1 phase checkpoint) and the next step begins.
Differentiated cells that do not divide (e.g., neurons or cells from the cardiac muscle) leave the cell cycle at this point and start the G0 phase[1].
Differentiated cells that do not divide (e.g., neurons or cells from the cardiac muscle) leave the cell cycle at this point and start the G0 phase[1].
▣ S: DNA is replicated. In this way, two daughter cells inherit the same genome from the mother cell.
▣ G2: repair phase of DNA and preparation for mitosis. The cell doubles its size compared to G1 phase.
Mitotic phase
The mitotic phase is a multistep process in which the duplicated chromosomes align, come apart and move to the two new daughters created.
The first stage of the mitotic phase is called karyokinesis, mitosis or nuclear division and the second step is known as cytokinesis, which is the physical separation of the cytoplasmatic components into the two daughter cells.
Mitosis, where the genetic material is divided equally, comprises the following stages:The first stage of the mitotic phase is called karyokinesis, mitosis or nuclear division and the second step is known as cytokinesis, which is the physical separation of the cytoplasmatic components into the two daughter cells.
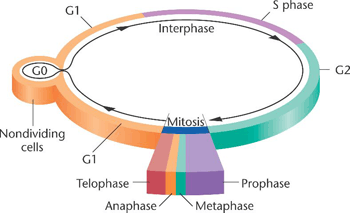
▣ Prophase: chromatin is condensed, the mitotic spindle[2] is formed and the nucleolus disappears.
▣ Prometaphase: the nuclear envelope is removed and the chromosomes join the microtubules through the kinetochores[3].
▣ Metaphase: the chromosomes align randomly in a plane called metaphase plate, or equatorial plane, between the two poles of the cell.
▣ Anaphase: the microtubules of the spindle become shorter, which causes the separation of the two sister chromatids.
▣ Telophase: the chromosomes decondense and the nuclear envelope, along with the nucleoulus is formed. Lastly, the mitotic spindle disappears.
During the last step of the mitotic phase, cytokinesis, a contractil ring of actin filaments is produced which divides the cytoplasm into the two daughter cells.
Control of the cell cycle
The duration of the cell cycle is higly variable, even in cells of the same organism. In human beings, it ranges from just a few hours in the embryonic cells, to two - five days for epithelial cells, to a whole human life for specialised cells, like cardiac muscle cells or cortical neurons.
The time that a cell spends in each phase of the cycle varies too. Thus, for example, those human cells with a 24-hour cycle spend approximately nine hours in the G1 phase, ten hours in the S phase, around four and a half hours in the G2 phase and about 30 minutes in the M phase.
Each step of the cell cycle is controlled by mechanisms both internal and external to the cell.
Regulation of the cell cycle by external mechanisms
Both the start and the termination of the cell replication cycle are activated by events external to the cell.
For instance, events such as the death of a near cell, the release of hormones that favour growth or the cell reaching a specific size can start cell division.
Regardless of the type of externally received message, a series of internal effects then lead it to the interphase.
From this starting point, each required parameter in each phase must be satisfied so that the cell cycle can continue.
For instance, events such as the death of a near cell, the release of hormones that favour growth or the cell reaching a specific size can start cell division.
Regardless of the type of externally received message, a series of internal effects then lead it to the interphase.
From this starting point, each required parameter in each phase must be satisfied so that the cell cycle can continue.
Regulation at internal checkpoints
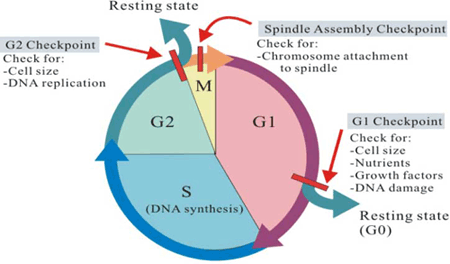
The checkpoints of the cell cycle prevent mistakes in the duplication or in the distribution of chromosomes (which can cause mutations).
We can distinguish three checkpoints:▣ The G1 checkpoint: also named the ‘rectriction point’ in mammals. It acts at the end of G1 phase and determines if all the necessary requirements are fulfilled to proceed the cell division.
 Si no se cumplen, la célula puede parar el ciclo hasta que las condiciones adversas se solucionen o puede pasar a la fase G0 y esperar señales que indiquen que las condiciones han mejorado.
Si no se cumplen, la célula puede parar el ciclo hasta que las condiciones adversas se solucionen o puede pasar a la fase G0 y esperar señales que indiquen que las condiciones han mejorado.
 Si no se cumplen, la célula puede parar el ciclo hasta que las condiciones adversas se solucionen o puede pasar a la fase G0 y esperar señales que indiquen que las condiciones han mejorado.
Si no se cumplen, la célula puede parar el ciclo hasta que las condiciones adversas se solucionen o puede pasar a la fase G0 y esperar señales que indiquen que las condiciones han mejorado.▣ The G2 checkpoint: carries out its role during the transition between G2 to M phases. Its most important function is to make sure that the chromosomes have been replicated and that replication has been accomplished without DNA damage. If damage is detected, the cycle stops and the cell tries to complete the DNA replication or repair the damaged one.
▣ The M checkpoint: is also known as the spindle checkpoint. It acts near the end of the metaphase stage of karyokinesis. It determines whether the sister chromatids are firmly and correctly bound to the spindle microtubules by the kinetochores.
The control system of the cell cycle could be compared to the program of a washing machine, where the washing machine only carries out its functions when the signals from its sensors (lock door, levels of water and detergent, power supply) indicate that it is ok to move forward during the diverse stages of the washing cycle.
Regulator molecules of the cell cycle
In addition to the checkpoints, there are some intracellular molecules that regulate the cell cycle and can act individually or affect the activity or production of other regulator molecules.
Regulator molecules are classified into two groups:
▣ Those molecules that promote the progress of a cell to the next phase (positive regulation). They are, in turn, divided into two types: cyclins[4] and cyclin-dependent kinases (CDKs)[5].
▣ The second group are negative regulators, which are in charge of halting the cell cycle. The best-known negative regulators are retinoblastoma protein (Rb), p53 and p21.
Retinoblastoma protein is a suppressor protein for tumours that is altered in many kinds of cancer (prostate, breast), but it is retina cancer from where it takes its name.
P21 inhibits the cell cycle, with its levels controlled, in turn, by p53, which regulates cell growth and controls DNA damage. This protein can halt the cycle if needed, and is able to cause apoptosis too.
Retinoblastoma protein is a suppressor protein for tumours that is altered in many kinds of cancer (prostate, breast), but it is retina cancer from where it takes its name.
P21 inhibits the cell cycle, with its levels controlled, in turn, by p53, which regulates cell growth and controls DNA damage. This protein can halt the cycle if needed, and is able to cause apoptosis too.
[1] Permanent or temporary quiescence (inactivity) phase in which the cell cycle stops.
[2] Set of microtubules whose function is to enable the migration and correct separation of the chromosomes during mitosis.
[3] Protein structures whose function it is to initiate, control and supervise the movement of the chromosomes during cell division.
[4] Proteins synthesised during the interphase and destroyed at the end of the mitosis. They regulate the enzymatic activity of CDKs.
[5] Enzymes that activate or inhibit other proteins by phosphorylating them (adding phosphate groups).
[2] Set of microtubules whose function is to enable the migration and correct separation of the chromosomes during mitosis.
[3] Protein structures whose function it is to initiate, control and supervise the movement of the chromosomes during cell division.
[4] Proteins synthesised during the interphase and destroyed at the end of the mitosis. They regulate the enzymatic activity of CDKs.
[5] Enzymes that activate or inhibit other proteins by phosphorylating them (adding phosphate groups).
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://www.biocancer.com/journal/1219/41-regulacion-positiva-del-ciclo-celular
http://www.pucmmsti.edu.do/websise/estudiante/materias/201120122/ST-BIO-112-T-003/04.%20Regulacion%20del%20ciclo%
20celular%20Apoptosis%20Cancer%20%20.pdf
http://bio3400.nicerweb.com/Locked/media/ch02/interphase.html
http://www.slideshare.net/obessionforu/cell-cycle-and-molecular-basis-of-cancer
http://biologicalexceptions.blogspot.com.es/2013_01_01_archive.html
http://gleesonbiology.pbworks.com/w/page/7537859/C9
http://www.bioscirep.org/content/30/4/243
http://www.biocancer.com/journal/1219/41-regulacion-positiva-del-ciclo-celular
http://www.pucmmsti.edu.do/websise/estudiante/materias/201120122/ST-BIO-112-T-003/04.%20Regulacion%20del%20ciclo%
20celular%20Apoptosis%20Cancer%20%20.pdf
http://bio3400.nicerweb.com/Locked/media/ch02/interphase.html
http://www.slideshare.net/obessionforu/cell-cycle-and-molecular-basis-of-cancer
http://biologicalexceptions.blogspot.com.es/2013_01_01_archive.html
http://gleesonbiology.pbworks.com/w/page/7537859/C9
http://www.bioscirep.org/content/30/4/243
Read more